Original Sujie Small Peanut Net

Author: Su Jie
Chinese teacher in Chaozhou High School, Guangdong Province
Chaozhou Daily’s Special Writer for College Entrance Examination Preparation
WeChat official account | Chinese guy; Film x science
Tao and technique of Chinese: the use of teaching materials and the art of taking exams, Absolute rationality: exploring the limitations of thinking.
"Exam-oriented ability is quality education!"
Can you accept this view?
Recently, Mr. Su Jie, a middle school Chinese teacher, who is listening to the wind in Huayou @ Takejian, threw out this view in community sharing and put forward 10 arguments in combination with the preparation for the drama "Dragon Cherry". And summed up the four characteristics of super teachers who can send "ordinary students" to famous schools.
Teacher Su Jie worked as a Chinese teacher in a high school in Chaozhou, and studied the preparation for the college entrance examination for many years. This article is also his thinking from the perspective of preparation.
Tips: "Dragon Cherry 1" is an enlightening Japanese drama for many people. It was first broadcast in 2005. The story about the college entrance examination was rated as "the sooner you watch the better Japanese drama", and Douban scored 8.8. Recently, the second film was released, and the score was still above 8.
The protagonist Sakuraki Kenni, a down-and-out lawyer and ex-mobster, went to teach a stupid senior 3 student with an average score of 36 in Longshan High School and was admitted to the first Tokyo University in Japan … "Long Ying" was listed as a candidate by many high school teachers to watch Japanese dramas.

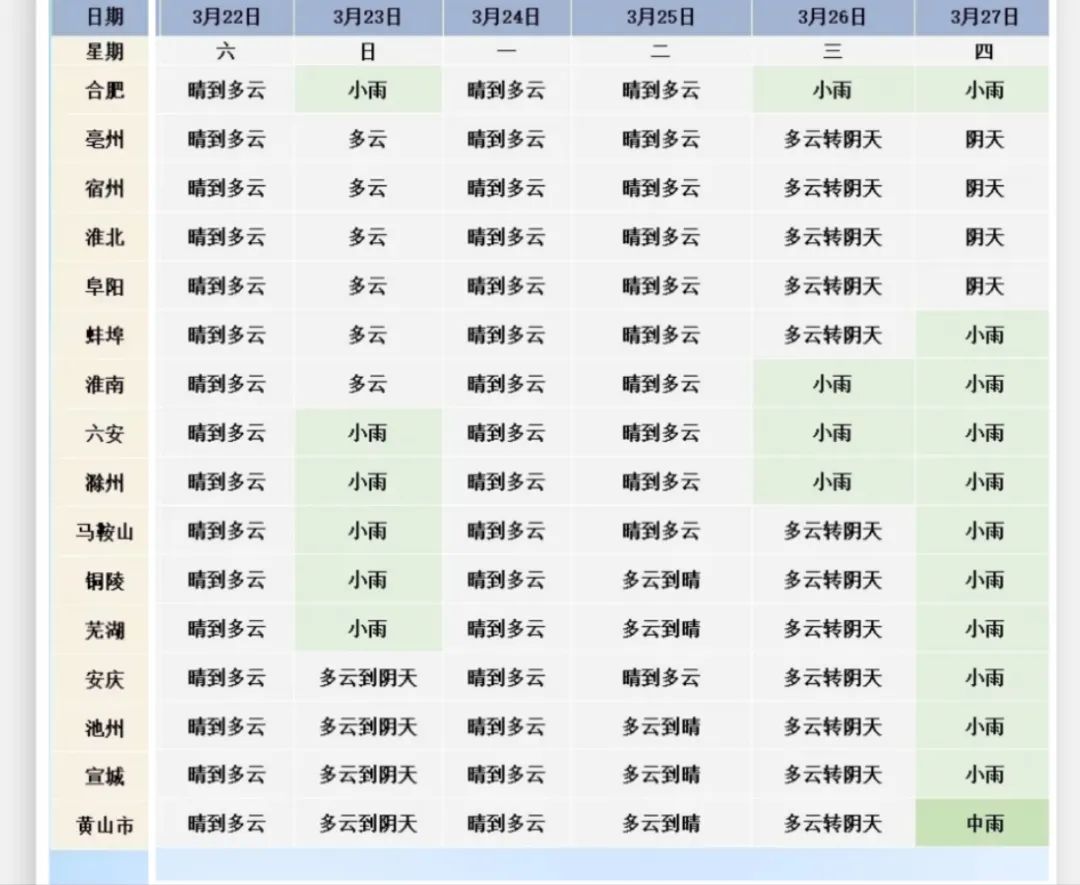
Teacher Sakuragi roared, "Idiots and ugly people go to Dongda!" The influence spread to the society. In the year of broadcasting alone, the number of cram schools in Japan soared by 12%, and the number of applicants for Dongda University soared by nearly 400.
When I saw the second season of the Japanese drama "Dragon Sakura", I found that more than ten years had passed!
As a TV series, to attract the audience, it’s natural to go on without making difficulties! In reality, for the Japanese, the University of Tokyo is equivalent to our Peking University Tsinghua. The school has a special class. If we leave it here, parents will do whatever it takes to cram their children in. As a result, this Japanese drama created a lot of contradictions, and only reluctantly increased the number of students in the special training class to seven (and finally added two more listeners).
Then, the story is: send some idiots to Dongda University!
Ok, that’s the end of the nonsense. A boring teacher like me is always looking at teaching! Moreover, it must be admitted that "Long Ying" written 15 years ago is very enlightening to my own teaching.

Many years ago, my teacher taught me a sentence: taking an exam is also an ability. Looking at "Long Ying 2", we can consolidate this understanding and make it clear that the ability to take exams is quality education!
From the 10 prerequisites for being admitted to Dongda University,
Talk about why "exam-oriented education is quality education"
Let’s first look at the many prerequisites for "being admitted to Dongda University" summarized by teacher Sakuragi, the hero of the film. Why do you say that-
1. The ability to think about the nature of the problem
Sakuragi said: What is the most attractive ability of Dongda University? Is how much ability to think about the essence.
The film also gives a realistic example, which is familiar to us and is the root of English:
To remember unite, you can find the root uni, which means "one". Then become a whole, so unite means unity. In addition, there are the meanings of combining, supporting, getting married and having both.
It’s easy to remember if you think that they are all derived from one etymology.
Uniform is a uniform costume for the team, university is a unity of various colleges and so on, unicorn is unique because it has only one corner.

This truth, in fact, is what Confucius said: a gentleman’s business is based on the foundation and the Tao is born.
In our words, the world is complicated, but behind it are basic elements and basic theoretical composition. Just as Marxist philosophy says: the world is material, the material is changing, the change is regular, and the law can be mastered!
If you are not satisfied, I want to review it. In the film, I also gave a real question (the geography question of Dongda University in 2015):
Please state the reasons for importing large quantities of pumpkins from Mexico and New Zealand. Plus, the season of pumpkin is from summer to winter. From this perspective, what do you think? Answer: There are only summer and autumn in Japan, but in Mexico, it is warm all year round. When it is winter in Japan, it is summer in New Zealand.
Back to the teacher’s analysis:
Everything has its meaning and essence. Why do you ask this question? Why do we ask you to do something that looks like fun at first glance? We should capture the essence from different angles. In fact, this question is very similar to the geography question of our college entrance examination in recent years.
2. Empathy
For students who are conceited and look down on others, Mr. Sakuragi said early in the morning that he failed the exam because:
Dongda often gives questions from multiple perspectives, that is to say, it can consider the feelings of people standing in all positions. They want such people, and this is the message from Dongda University. People who crowd out the people around them and just want to go up on their own are not what Dongda wants. So I said before that you can’t go to Dongda University. Fujii, people like you are not needed by Tokyo University and society. Think about it.

In short, it means empathy. Specifically, it also means understanding different people’s viewpoints and looking at problems more concretely.
The film also gives an example: Fujii lost to three scum in the competition, because the English test is:
What will happen if you can read other people’s hearts? Please write down your thoughts. You can use multiple short sentences to write.
Fujii’s answer:
If people all over the world can read each other’s hearts, then the world will usher in peace. This is because war will never happen again, and crime and injustice all over the world will gradually decrease. In addition, due to this change, the influence of the news industry will gradually decrease.
Super teacher’s evaluation is:
It’s too long, and I have no idea what you’re talking about.
Fujii retorted:
It’s easy to understand, because you can’t lie, so you will be peaceful.
Super teacher summary:
Well, I finally understand. Your answer lacks the key word "can’t lie", which is not sufficient and lacks theoretical support. Moreover, you overuse advanced words and expressions, which leads to many spelling mistakes.
The answers of the three scum, however, are all expressed in basic English because the content is easy to understand. Although there are spelling mistakes, the scores are higher.
Amano:
If I can read other people’s hearts, I can make many friends, what they like and why they feel happy.
Hayase:
If I can read other people’s hearts, I will be happy, because I can read what the teacher is thinking, so I can get good grades in the exam.
Iwasaki:
If I can read other people’s minds, I can win the game more smoothly, because I can read the opponent’s mind during the game.
In other words, the content is concise and the logic is clear. Of course, this is also consistent with the answering skills of the college entrance examination before us.
Similarly, let’s look at a question in the film (2010 Tokyo University Entrance Geography Test):
Taiwan Province people come to Japan mostly for sightseeing. Where do you think is popular as a tourist destination in Japan? Please answer the questions outside Nara, Kyoto. As long as the answer is reasonable and well-founded.
Dishes:
I think it’s Chiba, because Disneyland is there. Osaka is also good, because there is Universal Studios.
Ichiro:
I think it’s Hokkaido, because it doesn’t snow in Taiwan Province. I heard that the tourist attractions where snow can be seen are very popular.
Both of them got it right, as long as it is justified. After hearing this sentence "as long as it is reasonable and well-founded", we should also be familiar with the "what makes sense" in the Chinese reference answer of the college entrance examination.
Then, if we look at the topic carefully, we can also find out whether such a "pragmatic" question is the way of thinking that elites who serve the Japanese government and enterprises should learn.
How to provide targeted services for Chinese in places where there are many tourists in China? On the contrary, I just think I’m "worried about the world", but actually I can’t even stand on my feet logically.
3. Empathy ability
To have "empathy", we should put ourselves in the other’s shoes and put ourselves in the other’s shoes. When it comes to empathy, it is more about "thinking from another angle" than just thinking from the other side.
In the film, the problems of several stupid students mainly lie in their lack of reading and poor understanding. Poor understanding, so poor Chinese, poor Chinese, other subjects will not be good.
Sakuragi teacher said:
Listen, the reading problem of Dongda University is equality, contrast and causality. In strict accordance with these three structures.
The most important thing is equality, that is, conversion. And it’s not just in Chinese. For all subjects, the ability to convert statements is very important.
This needs to be explained in detail. What is referred to in the film should be practical texts that are mainly expository texts, while the college entrance examination in China is information texts that are mainly expository texts. We are mainly based on three structural frameworks of "juxtaposition, contrast and progressive", which can be found on page 184 of "The Tao and Technique of Chinese: Teaching Material Application and Examination-oriented Art".

In addition, the three structures mentioned in the film are actually the internal logical structures of concrete sentences. Chinese grammar includes: juxtaposition, progression, turning, causality, hypothesis, condition and so on.

Mizuno added a detailed explanation:
For example, mathematics is to expand the given formula and convert the given expression into numbers. It is a subject of equivalent transformation. Even if you remember the formula, you can’t answer the question without the ability to convert numerical values.
Science and society (equivalent to our previous literature and science, and now we are ahead of Japan) need to transform the knowledge and information learned from textbooks into the same form as "problem setting". Chinese and English, naturally, are both subjects that need to use the ability to convert words. For example, Japanese-English translation is a straightforward example.
Sakuragi added:
All disciplines need you to have the ability to convert this statement. That is to say, for example, in short, the so-called, in other words … use these related words to interpret the problem more, and the answer will naturally appear.
The topics raised by the East Congress are basically similar to the question of putting this sentence in another way. In other words, if you can have reading comprehension ability, all subjects will go to a higher level. The key to getting into Dongda University is reading comprehension.
In other words, students need to be familiar with this method of "changing expression" and "understanding from another angle and way", and learning it is the key to understanding. Like Chinese, as a tool of communication and thinking, it must be based on understanding. Mathematics and physics also need to switch between "thinking in images" and "thinking in abstractions". For details, please refer to page 393 of Absolute Rationality: Exploring the Limitations of Thinking.
4. Generalization ability
In fact, according to teacher Sakuragi’s explanation of comprehension ability at the beginning, it is generalization ability:
Reading comprehension is the ability to understand and summarize what the author wants to express, that is, the ability to briefly summarize, and you obviously lack this ability. I can see at a glance that you haven’t read much. How many books you have read since childhood determines your reading comprehension.
Parents tell stories, read books independently, investigate documents, etc. The more they treat these things as everyday people from an early age, the stronger their reading comprehension ability.
Therefore, when explaining the comprehension ability, in addition to "equivalence" (equivalent exchange), the play also talks about another method: subject predicate method.
It is to specifically describe who did what (see Chaozhou Daily’s June 14, 2021 "Chinese Core Method: Subject Expression Analysis" just explained in detail the extensive use of this method in the exam).
The stupid students in the film can’t accurately understand the content of the article, and they are asked to sum up the junior high school Chinese text "Run, Meles" in 100 words. They are all dizzy, so the teacher first helps to find out the subjects-characters and let them express them to string together stories.
Of course, such a topic also exists in China, and we call it "generalization".

Letting a group of scum go to Dongda University is an effort to make the "student quality" and "taking the exam for Dongda University" more corresponding.
However, Sakuragi has always attached great importance to the essence of students. Although the knowledge base (a class of scum) and intelligence are actually not decisive (the intelligence difference between students is not great).
But as you sow, you reap, and you need the seeds of a big tree to grow into a big tree. Therefore, in addition to showing the "examination requirements", the choice of several academic scum can also help us understand "how quality is reflected".
Sakuragi has always paid attention to whether students can make materials and what advantages they have, which corresponds to Dongda’s understanding requirements for elites. Therefore, the growth contradictions of several students actually spell out the complete "quality".
5. Ideal courage
For example, Hayase and Amano have courage and ideals. In the words of Sakuragi:
When we set up a special class in Dongda University, we were laughed at by all the teachers and students. Only Cai Xu and Koichiro stood up and said that they wanted to take an examination of Dongda University. There are only two people out of 645. People who can make contributions in the world are all like this. They have a strong will to break through no matter how difficult the road is. They have this will. They already have the most important thing to get into Dongda University.

In addition, Sakuragi also pointed out the "luck" of Hayase:
In an ordinary family, raised by ordinary parents, went to an ordinary school, it is this kind of ordinary that is lucky. Because there is nothing to bear, nothing to encounter, and nothing to be bound, but only love and support.
And Sakuragi’s point of view is:
For exams, luck is the most important thing. Anyone who understands the exam knows that powerful students are actually reducing the "probability of accidents", and it is not impossible to encounter Waterloo.
6. Motivation
For example, Seto’s classmate, whose parents died, lived alone with her sister and had to help in the noodle restaurant since she was a child. But also with family debts. This not only made him understand the embarrassing fate without knowledge, but also gave him the motivation to change his destiny urgently. This is a powerful motive that can support him! Although the foundation is the worst, it can stick to the end.

7. Competitive quality
For example, Iwasaki, as an excellent athlete, has competitive spirit and perseverance, is used to intensive training, and has enough competitive wisdom.
However, her weakness is that she is "used to accepting other people’s arrangements", because her parents have no chance to participate in the Olympic Games, so she was trained from an early age and hoped that she would become an Olympic athlete.
Therefore, she must learn to be her own master and choose her own destiny. When she can’t continue to The way of the Olympic Games, she should give full play to her advantages in learning, never admit defeat, and always explore the way to win.

8. Analytical ability
For example, Jiantai’s classmate, his grades are very bad, and on a five-point scale, almost all of them are 1 point, at most 2 points. And he can’t listen at all. However, he has extraordinary analytical ability and can accurately predict the weather through ants’ "closing the door".
Sakuragi asked him:
Why do you know it will rain at 10 o’clock?
He said:
Because the ants are closing. On March 23, 2019, at 12: 42 on Saturday, ants closed their nests and it rained at 2: 32 pm; At 6: 08 pm on Saturday, April 2, 2020, the ants closed the door and it rained at 8: 23; Ants’ tentacles or body hair are very sensitive to smell, sound and temperature, and they can feel the low pressure change caused by the temperature drop in the air, and will close the nest before it encounters a big flood.
The date, time and weather are all correctly remembered, and the rules are derived from the data. The observation, insight and memory are impeccable, and the ability to enter Dongda University is complete.

9. Ability to concentrate
Xiao Shan’s Chinese and English scores are excellent, and she is a bully among several academic scum. Her advantage is that the students collectively affirmed that Xiao Shan Ma Li should go to Dongda University, precisely because she has excellent concentration (we call it "concentration").
For example, people with excellent concentration have high quality of study and work. In other words, people who can concentrate on their studies and work are more likely to absorb more knowledge. Dongda is a knowledge base that can absorb all kinds of knowledge beneficial to society, so Dongda can gather active and concentrated people in society. In other words, Xiao Shan Ma Li should go to Neusoft to expand the possibilities in the future.

10. Comprehensive ability
As for the last classmate Fujii (who joined as a "willing to admit defeat"), there is no strong point in the film, but in fact he is also very focused, but more importantly, his personality is flawed, but as Sakuragi said, it can be corrected.
However, he is always the strongest comprehensive strength and has the best chance to go to Dongda University. Therefore, it will be chosen by the principal to challenge the Dongda special class of Sakuragi. Comprehensive strength is always the most important!

Super teachers who can send ordinary students to famous schools
Have four characteristics
Whether it’s a joke, it’s what many people have seen, that teachers are both nannies and bodyguards, or art, which over-exaggerates the hero’s ability; In reality, sending top students to a prestigious school is just a teacher from a high school. But if you really want to send ordinary students to famous universities, you really have to be super teachers!
Sakuragi in "Dragon Sakura 2" was created as a super teacher. Although our parents and teachers are not and can’t be super teachers, there is always a direction to set an example.
Why is it called a super teacher? Because we should not only know the exam, but also know the students; We must also understand communication, education and teaching. It seems that there are only five things, but it is actually very complicated.
Like the two students in the film, they have inherited their family business after graduating from high school. There is no cost problem in attending private schools. It is ok to be righteous, but it is really not good to study. Seeing that my friends can also sprint to Dongda University, I foolishly think that I will go to a famous private university like Waseda or Keio through hard work.
As a result, the super teacher told them: private universities only take three exams, which seems easy, but it is actually more difficult. English is very demanding, and the examination range is wide. The content of the exam is very uncommon. Reading a big book may only take a little test. Obviously, the college entrance examination in Japan is more about self-enrollment, so the test-taking strategy to be mastered is almost as complicated as that of art, sports and media students in China.
Ok, then just focus on one Dongda University! If you want to go to Dongda, you need to know what kind of students Dongda wants. This is like our syllabus. Now we have no syllabus for the college entrance examination, and the scope is equal to "syllabus+curriculum standards+background of the times+real questions for the college entrance examination".
But in fact, we need to know what core literacy, basic ability and necessary knowledge each subject needs. Super teachers have repeatedly said: this is the "most important" thing to take an examination of Dongda University!
We have explained this point in detail earlier. Now let’s take a look at how Sakuragi knows the students.
1. Know the students
What is "knowing students"? Of course, the first thing is to understand the background of the times (this is a bit like Lang Ping taking a new generation of women’s volleyball in Leap).
Mizuno also wants to use the "special training" method that Sakuragi taught them before (concentrated study for 16 hours a day), while Sakuragi said: The era of compulsory learning and one-way obedience has passed, and they have a new generation of values. First of all, they must understand this. And trust them on this premise.
What Sakuragi has done is "team learning", introducing two diligent and excellent students to become their benchmarks. Although stupid students will slack off, they will consciously study when they see those who are better than themselves, and they will consciously return to the classroom to continue their efforts.

Of course, "knowing students" also means that you need to be rich in common sense and know a little. When Sakuragi saw Iwasaki stealing chocolate from a convenience store and saw her fall down the stairs, she knew that she was in trouble. Just like herself, she was afraid of being expelled from the team because of meniscus damage, so she hid the truth of the injury and did something "exciting" to relieve stress.
Therefore, through his own life experience, "I gave up basketball because of meniscus injury, and I once became a punk, but I finally got growth through learning" to guide Iwasaki to determine his life goals.
Seeing such a "strange student" as Jiantai, I learned not only that he likes insects, but also that he loves them. So unlike Jiantai’s class teacher, in order to help Jiantai study, he made a lot of insect specimens to decorate the classroom, which frightened him.
Just because we know that Jiantai has excellent memory and analytical ability, and is interested in insect flying, we just gave him a full English paper on "Research on Insect Wings" by Colin Nei Lu, a Harvard professor, and an English dictionary.
Just relying on interest can make Jiantai improve the level of English, math and science at the same time.
Obviously, this requires super teachers not only to have rich common sense, but also to have rich experience. Why it is important to say that "leading by an expert" is because people who can achieve great things have their own legendary experiences and successful experiences.
Although the things that happen to everyone are different, they are essentially the same, so it is very important to guide them at critical moments.
2. Know how to communicate
Super teachers need high emotional intelligence and excellent communication and expression skills. Just as at the beginning, Mizuno took himself as an example and called on students to join the "Dongda Special Class", but everyone only cared about playing their own mobile phones and treated her as a joke. After all, for them, they were tired of drinking "chicken soup".
Sakuragi, on the other hand, did the opposite at first, deliberately saying "Don’t go to Dongda University" and lambasting the students as "stupid ugly girls", as if she were against Mizuno, but she soon focused on him and really listened to what he was going to say.
Sakuragi knows how to find the positive energy in the "enemy". For their "enemies", for example, at first, they thought that Sakuragi would take advantage of Jiantai, who had learning disabilities, and the class teacher who hired Xiao Shan just scared Sakuragi not to hurt her students. Huitou Jiantai was discovered to have extraordinary learning ability, which shocked her with a score close to perfect score in the math exam.
But sakuragi said to her:
When the child came to our class, he kept talking about the two of you, whether you are qualified or not, and devoted yourself wholeheartedly to this feeling, which has always been conveyed to the child. Because of you, for Jiantai, the school is a place where you can feel at ease. This is the most important thing that affects the development of talents.
In this way, I won good colleagues and friends.
For another example, from the very beginning, as an opponent of gambling-the principal who advocated quality education, when Sakuragi realized that the reason why she absorbed Jiantai was not because of "recruiting more students", but because of the principal’s feelings: there are all kinds of people in this world, and students will have to deal with all kinds of people in the future and survive. I hope students can learn to respect diversity and help each other. So even a child with developmental disabilities, as long as he wants to go to school, I will recruit him as an ordinary student.
Sakuragi also expressed heartfelt admiration for this:
The reason why Jiantai can display his talents is thanks to the free school spirit that you have created without compulsory learning. He has little sense of resistance and disgust to study. So once you start learning, you will learn knowledge at an amazing speed. Students should develop their individuality and grow freely. Learning can grow without coercion. But this does not mean that students are allowed to be free without discipline. People have the instinct to forge ahead. Once they realize it, students will break through the shackles and show their abilities. The teacher’s duty is to stimulate their sleeping curiosity.
For Kenta, although Sakuragi played a key role, he didn’t "be greedy for his own merits" and admitted that things were supported by many reasons. This is the real wise man. It is also really possible to "get more help."
3. Understand education
There are many people who understand education, and there are also many people who can say one, two, three, four. But in practice, it is difficult to do it properly. Education, like medical care, is ultimately a measurement and a matter of grasping the time.
Sakuragi’s educational strategy is actually very simple. It is to be good for students wholeheartedly, so that students can feel it sooner or later. However, to help students, we should not rush to teach, but wait for the opportunity, just like a ripe melon, everything has its own harvest season. This is also what Confucius said: no anger, no anger, no anger.
We need to observe and wait for the opportunity. Seto, for example, originally wanted to join the special class of Dongda University, but since his parents died, his family owed a lot of debts, and his sister borrowed usury and relied on a small noodle restaurant to pay it back.
Mizuno was ready to help early in the morning, but Seto refused, and Sakuragi stopped her. When Seto went to work, he was rejected (he was still a student, and according to the law, no construction site dared to accept him), and he was so-called "on his own", but the money for working was far from enough.
Only then did Sakuragi help him. Let him feel that he must "change his destiny by knowledge." Because in fact, "it is legally impossible to ask for the payment of interest in excess of the loan amount. Therefore, the elder sister who has already paid more than the payable amount does not need to pay more money. "They have already paid off their debts. It is only their lack of legal knowledge that keeps them being cheated by usury.
Only when we truly realize that learning is a success can we truly have the ability to solve problems, so that Seto can better devote himself to learning. When he feels powerless, he will help to solve it, and it will also make him understand "don’t refuse the help of others."
Sakuragi accidentally discovered that Iwasaki had stolen something and suffered a meniscus injury. He didn’t rush to communicate with her, but secretly bought the video of the convenience store and deleted it. When Iwasaki really made it clear that he was stupid in "covering up his pain" and that he couldn’t keep his sports career and felt helpless, he told her his life experience and let her know his real life goals.
When her handle was used by her partner to make a big fuss, hit her and usurp her university walk status; Even she misunderstood Sakuragi and even hurt her good friend almost to be sent to prison, feeling regretful and afraid, and then she was educated:
Now is such an era, no one knows what a video will be used for. Petty theft is such a thing. It is hard to give up once. You may think that it is just a chocolate, but that one can ruin your life. Badminton is the same. A guy who can’t follow the rules will lose his life.
Such a lesson is called entering the heart and lungs.
Although Hayase is lucky, she is also favored too much because of her luck, which also causes the problem of three-minute fever. Sakuragi waited until she couldn’t bear it and decided to give up before she reasoned with her:
Hayase, if you go on like this, you will let this luck slip away. People who don’t realize that they are lucky often end up in an unfortunate life … People who don’t realize their luck can’t be satisfied with the status quo and always pursue something, but they have no determination about it. So once you have a little difficulty, you will give up, so once you have a little difficulty, you will give up and pursue the next one. It always takes three minutes.
Luck, too, will come one by one, but your determination will get weaker and weaker. Do you think this kind of person will be happy? Do you admire Xiao Shan, but Xiao Shan is Xiao Shan and you are you. If you have the determination to be admitted to Dongda University, you won’t care about the eyes of people around you. If you want to be happy, you must make up your mind.
Because of the "three minutes" fever, someone must have said something about her, but only when she is faced with a key choice and a painful abandonment will this kind of discourse about "life direction" and "happiness" be heard.
For Fujii, Sakuragi knew from the beginning that he had a high opinion of himself, and two excellent brothers put too much pressure on him who had made mistakes in the senior high school entrance examination. His brother’s ridicule also caused him to be unable to sincerely look at others, look down on teachers who graduated from third-rate universities and look down on stupid classmates around him.
However, Sakuragi only hit him through the competition and frustrated him until he fully realized his stupidity-he lost to a special class he despised and lost to an idiot he once thought.
When he faced failure, he taught him a few words, and then finally gave him a step:
You are willing to "admit defeat" and come to join Dongda class!
4. Know how to teach
What is teaching? Teaching is a systematic project! Especially, exam-oriented teaching needs to be accurate, efficient and comprehensive.
The first is the cooperation of the family. Under the persuasion of Sakuragi, the two parents agreed to let their children join Dongda class. Sakuragi gave them a "Dongda qualified winning method; Ten family rules ":
1) Have breakfast together
2) Ask children to help with at least one housework: It is very important to take daily life seriously. When it comes to exams, spending all your time on study will make the family atmosphere abnormal, and children are sensitive. Once they feel that they have brought a burden to their families, they will feel guilty, which will lead to great pressure and will probably abandon the exams.
3) Moderate exercise
4) Let the children take a bath at the same time every day: form habits through regular life, and then stimulate learning to form a virtuous circle. When learning becomes a matter of course, victory is in the bag.
5) Don’t force yourself when you are unwell, and have a good rest.
6) Keep the living room clean and tidy at all times.
7) Don’t urge children to study
8) Maintain a good relationship between husband and wife
9) The family goes out for dinner once a month.
10) Share these ten rules with the father of the child.
Obviously, the content is very detailed, and each criterion has a specific reference and purpose.
And systematic education, parents have to cooperate, food can not be ignored. Not only told the students: pork is rich in nutrition and vitamin B1 to relieve burnout, and it is most suitable for the body tired from study. Eggs and spinach contain amino acids needed by brain neurotransmitters, and this egg roll can be said to be the canned spinach of candidates (the story of Popeye).
Food is very important to exercise the brain and physical fitness. If you want to get into Dongda University, you should pay attention to every meal. Then wait for the students to eat seriously and work hard, and then continue their education when they are full and sleepy: what will happen to people’s bodies if they eat food in a short time? Blood sugar levels have risen sharply. Yes, after the blood sugar level rises sharply, the body will secrete a lot of insulin, which is used to convert sugar into energy.
I feel sleepy and tired after a meal, which is the reason. Don’t eat until you are full, so as to prevent the sharp rise of blood sugar. Feeding should take into account the efficiency of brain energy consumption. In that way, you can ensure more time to concentrate on your studies. Buddhism has the requirement of doing homework when eating and sleeping, which is actually the same reason.
Secondly, before taking part in the first simulation test of Dongda University, the "six simulation test rules of Dongda University" were given:
First, refer to the Dongda simulation test 6 times a year (there are 10 times a year, and we have to take at least 6 times); The examination time of Dongda University is very long, and it is not uncommon for the examination time to exceed 2 hours according to different subjects. Therefore, you should have the willpower and physical strength to match it. Take the exam six times a year, just to get used to it.
Second, Chinese must start from classical Chinese. Chinese can’t be answered from the beginning in the order of questions. The most important thing to pay attention to in the exam is time allocation. How to answer questions efficiently is the key to success. Start with the questions that you can definitely answer correctly and get the score. This is classical Chinese.
Modern Chinese in Dongda language is very difficult. Comparatively speaking, some problems in classical Chinese and Chinese are simply translated into modern Chinese, which makes it easier to get points. Starting with a difficult modern text will get you stuck. And when you get easy classical Chinese, you will be anxious because of lack of time. Therefore, Chinese must start from classical Chinese.
Third, mathematics uses words to write ideas. Dongda Mathematics is as difficult as modern Chinese. I’m afraid some of you can’t write formulas to work out the answers. But if you don’t answer, you will get 0 points again. So you have to write ideas. Write down how you want to solve the problem like this. In this way, you can get a little score, and the score of Dongda Mathematics includes problem-solving steps. As long as they can see your thoughts, they will definitely score. No matter what, don’t hand in a blank paper. This is the law of Neusoft Mathematics.
Fourth, be sure to bring snacks. The examination of Dongda University lasted from 9: 00 a.m. to 7: 20 p.m., which was a protracted war. In particular, the Chinese exam is two and a half hours, which is a physical battle, so let you bring snacks.
Fifth, the society will only test the contents within the prescribed scope, that is, it will only show what we have learned, so there will be no modern problems in the model test in June (it is estimated that students have not learned there in June), so there is no need to prepare.
Sixth, don’t take notes during the listening test. Look at the English test paper distributed to you and take notes while listening to the tape. It means yes, that’s a trap for you. Do you like sushi? Listening test is just like sushi, it pops up one after another. If you want to take notes, write down everything you hear, and focus on your notes, it is easy to miss important content.
One more thing, be sure to read the questions and options before listening. Predict in advance what will be put in listening, and it will be easier to remember then. To get into Dongda University, we should use all the known information flexibly.
Although it is the law before the simulation, it is actually the same as what our teachers told us before the college entrance examination. However, I have seen the campus of Chaoyang Experimental School, a famous college entrance examination school, and it is the "examination notes" posted on several walls. These rules, if they go deep into habits, will really help students a lot.
Thirdly, there are various strategies for taking exams and applying for exams:
We are all familiar with this, for example, how to adjust our goals in order to test Dongda University, after all, choose a good school, choose a famous school first and then choose a major; Choosing an ordinary school is the preferred major, and then choosing a school. Therefore, super teachers require students to do what they can.
Before the exam, Sakuragi put forward the "five points for attention in the college entrance examination":
1. Before the next day’s exam is over, you are not allowed to recall the subjects that have been tested.
What happens when you realize mistakes in other subjects in the exam? Will be shaken, you listen carefully, even if only a small mistake, when you remember this in your heart, people will be very uneasy; This is especially true if you think that this is a formal exam and there is no way out.
When you have a rest at noon, put on headphones to listen to songs and isolate the outside world. After the first day’s exam, all the subjects you took that day will be forgotten.
2. Don’t dwell on difficult problems
There are often difficult questions in one or two subjects in the college entrance examination, but don’t worry, the questions you find difficult are also difficult for others. The average score of that subject will be lower, so the person who can adjust his mood early will win.
The college entrance examination has a huge amount of questions, and it may be possible to write them if you think about them fully, but you have no time at all. You should give priority to the questions that can be sure to get the score. Start with the questions you can do.
Go home alone after the exam on the first day.
Listen, if you meet your friends, you can feel how they did in the exam without asking each other. Sometimes, because of such a trivial matter, you all become uneasy. You are forbidden to contact each other.
Be sure to write the answer on the test paper.
Anyway, it’s all multiple-choice questions. Just put a wreath on the numbers. This is to answer the questions immediately after the exam. It is closely related to the second test, that is, the entrance examination of Dongda University. According to the college entrance examination, you should submit the application form for the recruitment of Dongda University. In order to avoid your elimination, it is sometimes necessary to adjust your volunteer major according to your scores.
But when you got the results of the college entrance examination, it was after you submitted your college entrance examination volunteers. That is to say, you must assess the points yourself. Consider the level of the score line and submit the volunteer letter. That’s why you have to write down your answers. We can aim at majors with few competitors.
Taking the exam is the time to get along with yourself.
What others do has nothing to do with you. As long as you can pass the exam yourself.
These contents seem to be different from ours, but in fact, the essence and specific measures are consistent. Choose your own direction and adjust your strategy according to the probability of admission. Therefore, at the last minute, Sakuragi persuaded Fujii to change science to liberal arts, because as a liberal arts student, his mathematics and science are high enough to have an advantage in the competition among liberal arts students; If you continue to take the science exam, you will be kicked out with great probability.
Obviously, these contents are more related to the "psychological" level. As we knew from the beginning, both Sakuragi and Mizuno are lawyers, and they both take students as consultants and guide them to prepare for the exam (specifically, there are specially appointed subject teachers performing teaching). More like our head teacher, so we can also see how important it is for the head teacher to understand psychology.
The mastery of psychology comes not only from knowledge theory, but also from experience. As Sakuragi used to persuade the parents of Hayase and Amano:
You said that children "how can they get in the exam and fail it?" That is to say, you don’t trust your children. What can destroy children’s strong will is parents’ denial. Teenagers’ discussions with their parents almost all end in rejection.
In addition to the above general rules, in fact, the play also shows many specific teaching methods, such as:
Method 1. Learn from each other:
Amano is in charge of math, Hayase is in charge of Chinese, Iwasaki has the highest English score, Seto, you can’t do all subjects. But what’s the point of teaching each other only by fools? Because you are all idiots, you have unique advantages. If the person who teaches you is only a little better than yourself, your brain will think that you can do it without authorization (subconsciously). Moreover, in this way, people who teach can also organize knowledge, and only know the general part will be more clear and understand it to a higher level.
Method 1. Communicate with foreigners:
Have you started playing You Tube and Twitter? (Training English expression and courage)
Method 3. Integrate real questions into daily life:
Tomorrow, before and after you come to school, Mizuno will give you questions (actually, it is a real exam question, which seems to be a game, but training thinking will make students inadvertently familiar with the exam mode of Dongda University).
Method 4. Establish the ideal of life:
Finding the ideal is also finding yourself. It’s hard for students to persist, so if they want to persist, they must be given a firm future. This is also the familiar "sunshine in the heart and strength at the foot".
I made it clear that I don’t have much chance to participate in the Olympic competition. Iwasaki finally determined:
Let me be admitted to Dongda University. I want to study sports medicine in Dongda University. Then resurrect as a player and take part in the Olympic Games. Sports medicine is used to support young players after their retirement. This is my life plan now.
Kenta thinks:
For nature, human beings are harmful, destroying ecology and killing innocent people. This is not right. All life is equal.
Sakuragi said to him:
If you really think so, create such a world. To change society, to change common sense, to create a future that can coexist with insects.
Kenta asked how to do it?
Sakuragi told him:
Become a scholar, maybe you can do it. I’ll send you to Dongda University, and then it’s up to you.
Ultimate problem
How to face mistakes and even failures
However, in fact, the script also implies a problem, a difficult problem that may not be solved by the screenwriter or any educational expert. It is true that exams can test quality, and exam-oriented education can be quality education.
However, we have actually seen a problem of "doing everything possible and working hard" to win the final victory. High-pressure and high-intensity training, strong willpower to achieve a certain goal, when approaching the goal, there will be greater "resistance", which is easy to cause psychological damage and even personality damage.
So, we see the truth that Sakuragi made by shooting against Seto:
The reason why you failed at the critical moment is that you are too obsessed with every goal. The reason why I won was that I only planned to score 60% from the beginning, and I was prepared to score as long as I could, so I was not anxious when I didn’t score two goals at the beginning.
But you are different. You thought you won when you scored two goals at the beginning. Then the fifth ball missed, and your mind should be that you can’t lose the ball again, and all the rest should be won. You can’t win if you waver.
If you set up a good strategy at the beginning, you won’t be upset. This is the watershed of victory and defeat!
However, as long as they are people, they will definitely feel uneasy in front of the key finals. People who are fully prepared to work hard are even more. Control your uneasy mood! The strong players in the final realized that they were anxious and prepared for mistakes.
Listen carefully, don’t pursue perfection, and keep calm in the exam. The real strong will always imagine their own success, and then believe in themselves!
It’s really inspiring and inspiring. The question is, can you face failure? As the film tells at the beginning, why did Sakuragi leave school and hide is because a student who failed the exam tried to commit suicide, even though Sakuragi stopped him in time and was injured. But in fact, Sakuragi is deeply introspected about his successful education.
Then another student sent to Neusoft by himself, but in the end, he collaborated with the student who attempted suicide to deal with Sakuragi. Obviously, if you try your best to achieve your goals, and put this habit in other fields, will it create another terrible future elite?
If you just muddle through by ambition and skill, then such an exam will create scum instead?
I didn’t enjoy it
Welcome interested friends to discuss in depth.
See you in the comments section!
Original title: "Don’t take the chicken soup route, this" college entrance examination drama "has refreshed my understanding of exam-oriented education! 》
Read the original text